At this year’s K Trade Fair, ZAHORANSKY will clearly demonstrate its technological leadership with highly automated and fully integrated facilities in the medical technology sector.
“The future of manufacturing consists of lean production with integrated processes, as well as reduced interfaces with high availability and quality. We offer our customers complete turnkey production facilities with our one-stop-shopping approach. All injection molding, automation, and assembly processes are integrated there. We will be presenting solutions for this at the K Trade Fair.”
Robert Dous, Managing Director ZAHORANSKY Automation & Molds und Chief Sales Officer ZAHORANSKY GROUP
Integrated Injection Stretch Blow Molding (ISBM)
One focus at the booth will be on modern processes such as integrated Injection Stretch Blow Molding (ISBM). Thanks to the transfer handling integrated into the injection molding tool, ISBM makes it possible to produce vials on a standard injection molding machine – the special injection blowing machines that would otherwise be required are no longer necessary. The featured exhibit is also equipped with the new Z.LODOS tray loader. The special feature: The tray change time is “0”, meaning there is no system downtime required for the fully automatic tray change. This results in a significantly leaner production process in the manufacture of medical products, as well as easier compliance with cleanroom specifications. Another focus of the trade fair presentation is the expanded range of hybrid components, especially in the medical technology sector. In this area, plastic is used as a proven substitute for glass in pre-filled disposable syringes as drug dispensers, among other things. These products can be manufactured in one step using ZAHORANSKY’s highly automated large-scale facilities, reducing manufacturing steps and thus costs.
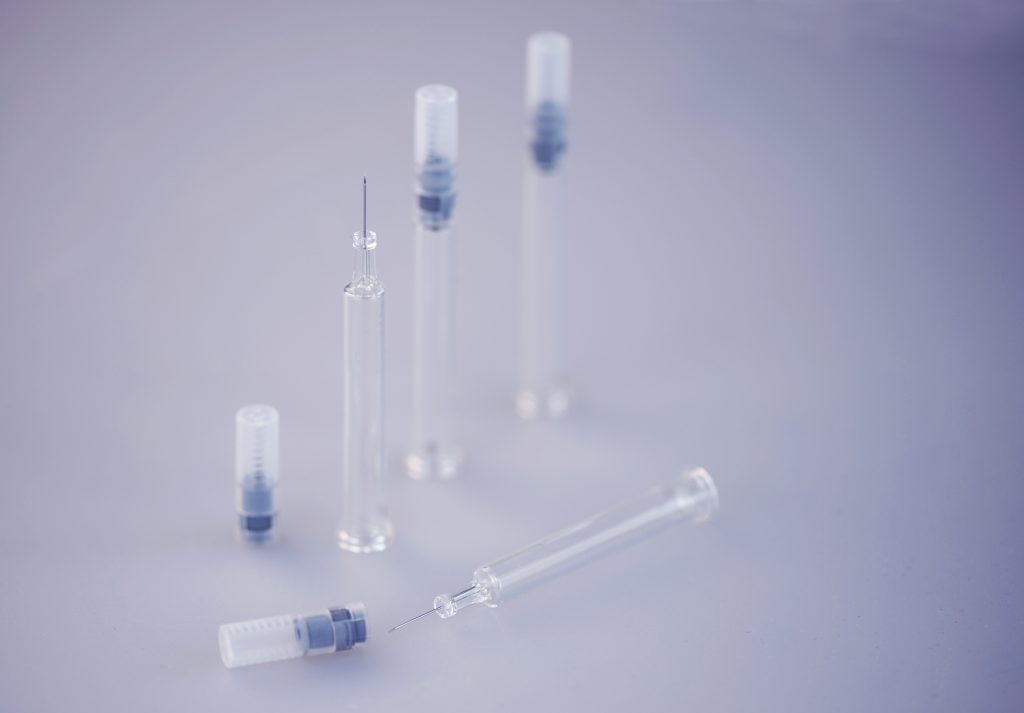
The PRIMA Z Syringe: The first production line on the market featuring a 16-cavity mold for manufacturing syringes
At booth E70 in Hall 1, ZAHORANSKY will show not only a selection of exhibits from hybrid component manufacturing, but also complete installations. The PRIMA Z Syringe, for example, is the first production line on the market featuring a 16-cavity mold for manufacturing syringes as primary pharmaceutical packaging for liquid medicines. These parenteral syringes, as they are known, are produced in a modern plastic injection molding process with overmolded cannulas. Compared to glass syringes, the plastic staked-needle syringes can be fed directly to be filled with the respective drug. With production systems such as the VITRO Z models, ZAHORANSKY can meet the growing global demand for in-vitro diagnostic products (IVD), such as pipette tips, cuvettes, laboratory consumables, and blood sampling tubes. Here, too, plastic is used universally as a substitute for glass. This enables these products to be manufactured in a short time, with high quality, short start-up times, and little waste.
O.K. status ensured throughout
The risk of malfunctions and defective parts is minimized in all facilities through continuous quality and process controls. Depending on the product and requirements, this can be guaranteed by 100% camera inspections, integrated X-ray modules, and leak tests. Even at maximum production utilization, such as the daily manufacture of 600,000 corona virus vials at the US company SiO2, the O.K. status of parts and components is ensured at all times.

Manufacturing 4.0
By using the digital twin, ZAHORANSKY can simulate the manufacturing and assembly of a variety of products – such as pre-filled syringes, various drug dispensers, and electric toothbrushes – in advance and calculate their cost-effectiveness. This reduces the time to market as the digital processes can be optimized in advance and then quickly transferred to the actual machines. The decoupling of functional units also increases the Overall Equipment Effectiveness (OEE).
Plastic in medical technology: the ideal glass replacement material

Plastic is the ideal alternative to glass, particularly in medical products such as pre-filled syringes, due to its material properties and advantages in processing and handling. In the manufacture of these hybrid components, such as pre-filled syringes with pre-filled medication, instead of risky bonding processes with drying and cleaning times, tried-and-tested processes are used where the needles are overmolded in a process-safe manner. This also makes it possible to manufacture inhalers and interdental brushes, as well as to overmold electronic components. This one-step production approach reduces not only the total number of production steps but the costs as well.



